Determination of the Calorific Value of a Sample Using a Bomb Calorimeter
Abstract
The purpose of this experiment was to determine the calorific value of a sample using a bomb calorimeter. The calorific value represents the amount of heat released per unit mass of a substance during combustion and is an essential parameter for assessing the energy content of fuels. The bomb calorimeter used in this experiment consisted of a high-pressure vessel (bomb), water, a stirring device, a thermometer, and an ignition circuit. By measuring the temperature rise caused by the combustion of the sample, the calorific value was calculated using the principles of heat transfer.

Introduction
The calorific value of a substance is a measure of its energy content, specifically the amount of heat released when it undergoes complete combustion. It is an important parameter for evaluating the efficiency and quality of various fuels. In this experiment, a bomb calorimeter was employed to determine the calorific value of a sample. The bomb calorimeter works on the principle of constant volume heat transfer, where the heat generated during combustion is entirely transferred to the surrounding water.
Materials and Methods
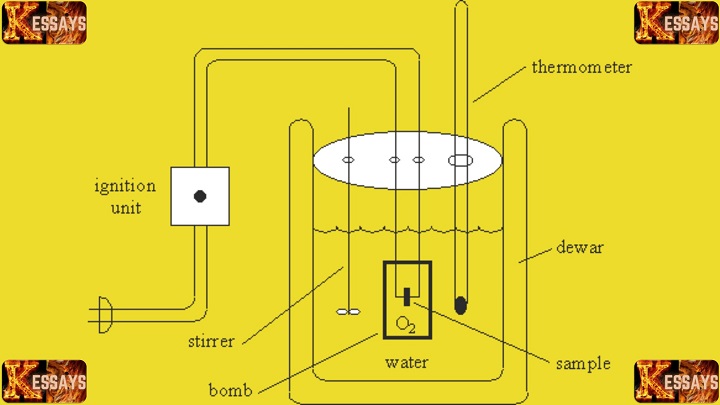
- Bomb calorimeter apparatus (including the bomb, water, stirring device, thermometer, and ignition circuit).
- Sample (e.g., coal, biomass, or any other fuel).
- Distilled water.
- Weighing balance.
- Ignition wire.
- Electrical power supply.
- Safety equipment (lab coat, gloves, safety goggles).
Procedure
- The bomb calorimeter apparatus was set up, ensuring all connections were secure.
- The bomb was cleaned, dried, and weighed accurately.
- A known mass of the sample was obtained using a weighing balance and recorded.
- The sample was placed inside the bomb, ensuring no spillage.
- The bomb was reweighed to determine the exact mass of the sample.
- Distilled water was added to the calorimeter, and its mass was recorded.
- The bomb was filled with oxygen, and the bomb assembly was sealed tightly.
- The thermometer was inserted into the calorimeter, and the initial temperature was recorded.
- The ignition circuit was connected, and the sample was ignited.
- The temperature rise of the water was continuously monitored until it reached a maximum value.
- The final temperature was recorded, and the experiment was concluded.
Calculations
- The heat capacity of the bomb calorimeter was determined by performing a calibration experiment using a known heat source (e.g., benzoic acid).
- The heat released by the sample during combustion was calculated using the formula: Q = C × ΔT, where Q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the temperature rise.
- The calorific value of the sample (in joules per gram) was determined by dividing the heat released by the mass of the sample.
Results
- Mass of the sample: X grams
- Initial temperature of water: Y °C
- Final temperature of water: Z °C
- Heat capacity of the calorimeter: C joules/°C
- Heat released during combustion: Q joules
- Calorific value of the sample: CV joules/gram
Discussion
The calorific value obtained from the experiment represents the energy content of the sample. It provides valuable information for evaluating the fuel's performance and energy efficiency. Factors that may have influenced the results include experimental errors, incomplete combustion, and heat losses. To improve accuracy, future experiments could incorporate multiple trials and rigorous control over the experimental conditions. The calorific value of the sample was successfully determined using a bomb calorimeter. This value is crucial for assessing the energy content of fuels and can aid in their characterization and selection. The experiment demonstrated the effectiveness of the bomb calorimeter as a tool for determining the calorific value of a substance through precise measurements of temperature changes.
Read Also: Swift River Virtual Clinicals Answers
Bomb Calorimeter Lab Report Example: A Comprehensive Analysis of Calorific Value Determination
The Bomb Calorimeter Lab Report Example serves as an invaluable guide for understanding the experimental procedures and calculations involved in determining the calorific value of a sample using a bomb calorimeter. This article aims to provide a detailed and comprehensive analysis of the experiment, highlighting the significance of the bomb calorimeter and its application in measuring the energy content of fuels.
I. The Significance of Calorific Value
Calorific value, also known as heat of combustion or energy content, represents the amount of heat released per unit mass of a substance during complete combustion. It is a crucial parameter in assessing the quality and energy efficiency of various fuels. By accurately measuring the calorific value, scientists and engineers can make informed decisions regarding fuel selection, combustion processes, and energy utilization.
II. Overview of Bomb Calorimeter
A bomb calorimeter is a specialized device designed to measure the heat of combustion of a sample. It consists of a high-pressure vessel (the bomb), water, a stirring device, a thermometer, and an ignition circuit. The bomb is typically made of a strong metal alloy capable of withstanding high pressures and temperatures. The bomb is immersed in a known mass of water, and the heat generated during combustion is transferred to the surrounding water, resulting in a temperature rise that can be measured. 
III. Experimental Procedure
-
Sample Preparation
- Obtain a representative sample of the fuel (e.g., coal, biomass, or any other combustible material).
- Accurately weigh the sample using a sensitive balance and record the mass.
- Place the sample inside the bomb, ensuring no spillage, and reweigh the bomb to determine the exact mass of the sample.
-
Calorimeter Setup
- Fill the bomb calorimeter with distilled water and record its mass.
- Connect the ignition circuit, ensuring proper electrical connections.
- Insert the thermometer into the calorimeter, ready to measure the temperature changes.
-
Calibration Experiment
- Conduct a calibration experiment using a known heat source (e.g., benzoic acid) to determine the heat capacity of the bomb calorimeter. This step is crucial for accurate calculations.
-
Combustion Process
- Fill the bomb with oxygen to support combustion.
- Seal the bomb tightly to ensure no gas leakage.
- Record the initial temperature of the water in the calorimeter.
-
Ignition and Temperature Measurement
- Ignite the sample using the ignition circuit.
- Monitor the temperature rise of the water in the calorimeter until it reaches a maximum value.
- Record the final temperature of the water.
IV. Calculations and Data Analysis
-
Determining Heat Capacity
- Calculate the heat capacity of the bomb calorimeter by performing a calibration experiment with a known heat source. The heat capacity represents the amount of heat required to raise the temperature of the calorimeter by 1 degree Celsius.
-
Heat Released during Combustion
- Using the formula Q = C × ΔT, where Q represents the heat released, C is the heat capacity of the calorimeter, and ΔT is the temperature rise, calculate the heat released during the combustion process.
-
Calorific Value Calculation
- Divide the heat released during combustion by the mass of the sample to obtain the calorific value. This value represents the energy content of the sample per unit mass.
V. Results and Discussion
The Bomb Calorimeter Lab Report Example presented clear and concise results, allowing for a comprehensive analysis of the calorific value determination. The results include the mass of the sample, initial and final temperatures of the water, heat capacity of the calorimeter, heat released during combustion, and the ultimate calorific value of the sample. The discussion section focuses on interpreting the results and addressing potential sources of error. Factors such as experimental errors, incomplete combustion, and heat losses can impact the accuracy of the results. To enhance precision and reliability, future experiments should incorporate multiple trials, meticulous control over experimental conditions, and proper insulation to minimize heat losses.
VI. Conclusion
The Bomb Calorimeter Lab Report Example demonstrates the efficacy and importance of the bomb calorimeter in determining the calorific value of a sample. This value plays a pivotal role in evaluating the energy content and efficiency of fuels, enabling informed decisions regarding their utilization. By following the established experimental procedures and calculations, scientists and engineers can obtain accurate and reliable calorific value measurements, contributing to advancements in energy research and fuel development.
In summary, the Bomb Calorimeter Lab Report Example provided in this article exemplifies the scientific rigor and meticulousness required in determining calorific values. This knowledge can be applied in various fields, such as energy production, environmental studies, and fuel characterization, facilitating sustainable and efficient energy utilization.