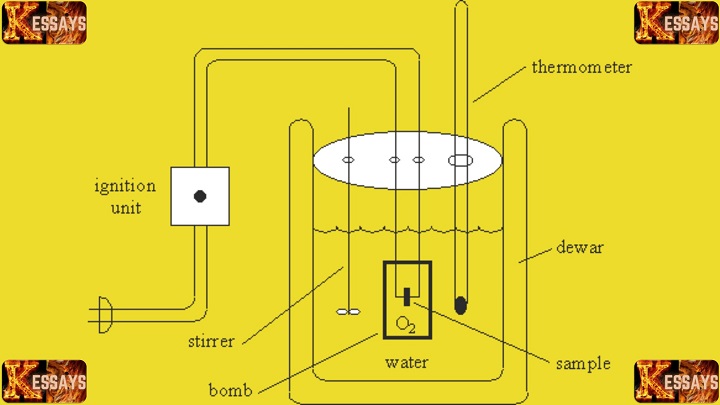
Introduction to Calorimetry Lab Report:
The Calorimetry lab report is a scientific odyssey into the realms of heat transfer, meticulously charted within the confines of a controlled environment, orchestrated by the unassuming yet powerful instrument — the calorimeter. This experimental journey is not a mere exploration; it's a deliberate quest to unravel the intricate principles governing heat exchange during
chemical reactions or physical processes. As we embark on this scientific endeavor, the Calorimetry lab report emerges as more than a record of measurements; it is a key to unlocking the secrets of energy transformations.
Delving into the Core Principles
At its essence, the Calorimetry lab report is a conduit for understanding the fundamental principles that dictate the flow of heat. It delves into the very fabric of thermodynamics, deciphering the language written in temperature changes and energy exchanges. Each observation, meticulously recorded within the controlled environment of the calorimeter, contributes to a broader comprehension of these underlying principles.
From Theory to Practice: Applications Across Disciplines
Yet, the significance of the Calorimetry lab report extends beyond the theoretical realm. It bridges the gap between abstract thermodynamic principles and real-world applications. The insights gained from this exploration find practical applications across diverse scientific disciplines. From chemistry labs to environmental science studies, the Calorimetry lab report becomes a versatile tool, offering a deeper understanding of the energy dynamics that shape our physical world.
Multifaceted Purposes: Unraveling the Unknown
As we navigate the complexities of the Calorimetry lab report, it becomes apparent that this
scientific pursuit serves multifaceted purposes. It is not merely about recording numbers; it's about unraveling the unknown. It's about peeling back the layers of energy transformations and deciphering the intricacies that govern the behavior of matter. The Calorimetry lab report, in its essence, is a key to unlocking the secrets of heat measurement and, by extension, understanding the very essence of thermodynamics.

The Purpose of the Calorimetry Lab:
At the heart of the Calorimetry lab report, there exists a profound and multifaceted purpose that transcends the mere quantification of heat changes. This scientific endeavor ventures beyond a routine measurement exercise, assuming the role of a systematic exploration into the intricate energetics entwined with chemical reactions and physical transformations. The essence of this purpose lies in the meticulous examination of heat exchange phenomena, a task made possible through the strategic use of a calorimeter.
Systematic Exploration: A Holistic Approach
The purpose of quantifying heat changes extends beyond the isolated goal of obtaining numerical values; it embodies a
holistic approach to understanding the thermodynamic behaviors governing diverse processes. Researchers embark on a systematic journey, unraveling the complexities of energy transformations within a controlled environment. This systematic exploration involves not only capturing the quantitative aspects of heat exchange but also deciphering the underlying principles that dictate the observed phenomena.
Precision Through Calorimetry: A Strategic Tool
The selection of a calorimeter as the instrument of choice underscores the strategic nature of this purpose. A calorimeter, with its ability to isolate a system and meticulously measure heat changes, becomes the linchpin in this scientific pursuit. It serves as the gateway to precision, allowing researchers to delve into the intricacies of chemical reactions or physical changes, armed with the capability to precisely capture the heat exchanged during these processes.
Illuminating Thermodynamic Behaviors: Insights Gained
Through the lens of calorimetry, researchers gain invaluable insights into the thermodynamic behaviors that underpin various phenomena. The calorimeter acts as a metaphorical flashlight, illuminating the pathways of energy transfer and transformation. This illumination extends not only to the quantitative aspects of heat changes but also to the qualitative understanding of how energy behaves within a closed system.
Synthesis of Insights: The Core Purpose Redefined
In synthesis, the core purpose of the Calorimetry lab report is a nuanced amalgamation of systematic exploration, precision-driven measurements, and the illumination of
thermodynamic principles. It transcends the boundaries of a conventional laboratory exercise, evolving into a scientific quest that deepens our comprehension of energy dynamics within the realm of chemical and physical transformations. As researchers venture forth, armed with a calorimeter and a purpose steeped in exploration, the Calorimetry lab report becomes a testament to the relentless pursuit of scientific understanding.
Key Components and Objectives:
Crucial Assumptions and Potential Errors:
In the intricate landscape of the Calorimetry lab report, acknowledging crucial assumptions and potential errors becomes paramount. As scientists embark on this investigative journey, the clarity of ideal conditions, interwoven with a keen awareness of potential pitfalls, shapes the narrative of precision and interpretation.
Crucial Assumptions: Setting the Ideal Stage
The Calorimetry lab operates under specific assumptions, delineating the ideal stage for precise measurements. These assumptions create a framework that allows researchers to unravel the mysteries of heat exchange without unnecessary interference. Acknowledging these assumptions becomes fundamental, as they set the groundwork for the controlled environment needed for meaningful insights.
Potential Errors: Navigating the Pitfalls
Simultaneously, an awareness of
potential errors introduces a layer of realism into the experiment. Incomplete insulation within the calorimeter, variations in ambient conditions, or even the minutest discrepancies in measurement instruments can introduce deviations. Recognizing these potential pitfalls is not a testament to failure but a proactive stance towards refining the experiment's accuracy.
Enhancing Result Interpretation: The Role of Assumptions and Errors
Understanding these assumptions and potential errors becomes instrumental in result interpretation. It transforms the experiment from a pursuit of perfection to a nuanced exploration, where deviations are not anomalies but stepping stones for improvement. By acknowledging the limitations inherent in any scientific endeavor, researchers elevate the interpretation of results beyond a mere quest for accuracy to a continuous refinement process.
The Calorimetry lab report becomes a journey of precision amidst realism, where assumptions and potential errors coexist. The deliberate setting of ideal conditions and the candid acknowledgment of potential pitfalls create a holistic narrative of
scientific exploration. Through this dual lens, researchers not only achieve meaningful insights into the energetics of chemical reactions or physical changes but also contribute to the ongoing refinement of scientific methodologies. The Calorimetry lab, with its acceptance of assumptions and vigilance towards errors, emerges as a testament to the dynamic and evolving nature of scientific inquiry.
The Heart of the Matter: Abstract and Results:
In the intricate tapestry of the Calorimetry lab report, two integral components emerge as the heart of the matter — the abstract and results. These sections, distinct in their purposes, work in tandem to encapsulate the essence of the experiment and present a numerical narrative of the observed heat changes.
Abstract: A Concise Prelude
The abstract stands as a concise prelude to the Calorimetry lab report, offering a sneak peek into its fundamental elements. It serves as a roadmap, navigating readers through the experiment's objectives, methodologies, and paramount findings. In its brevity, the abstract encapsulates the very soul of the scientific inquiry, providing a quick but comprehensive understanding of what awaits within the report's pages.
Results: The Quantitative Tapestry
Conversely, the results section unfurls the quantitative tapestry woven during the experiment. It goes beyond the abstract's preview, laying bare the numerical data acquired through meticulous measurements. Here, the heat changes observed take a numerical form, offering a quantitative account of the experiment's outcomes. The results section transforms the abstract's concise promises into a detailed numerical narrative, providing the foundation for in-depth
analysis and interpretation.
A Symbiotic Relationship
Together, the abstract and results sections form a symbiotic relationship, where the abstract sets the stage, and the results take center stage. The abstract tantalizes curiosity, inviting readers into the experiment's world, while the results substantiate the promises made, grounding the scientific inquiry in tangible data. This collaborative dance between brevity and depth, between preview and revelation, renders the Calorimetry lab report a holistic document, inviting readers to engage with the experiment on both conceptual and quantitative levels.
The abstract and results of the Calorimetry lab report emerge as the dynamic duo, each playing a pivotal role in conveying the experiment's intricacies. As readers traverse from the abstract's succinct overview to the results' numerical panorama, they embark on a journey guided by both
conceptual understanding and quantitative rigor. The heart of the matter, encapsulated in these sections, breathes life into the scientific narrative, ensuring that the Calorimetry lab report stands as a testament to meticulous inquiry and meaningful discovery.

Navigating the Lab Report Landscape:
To navigate the intricacies of the Calorimetry lab report effectively, students can seek guidance from
reputable assignment help websites such as kessays.com and
peachyessays.com. These platforms provide valuable insights, exemplary samples, and expert assistance, empowering students to craft well-structured, insightful analyses of their Calorimetry lab experiments. As we embark on the journey through this guide, the focus remains on unraveling the nuances of calorimetry, guiding students toward a comprehensive understanding of this indispensable scientific technique.