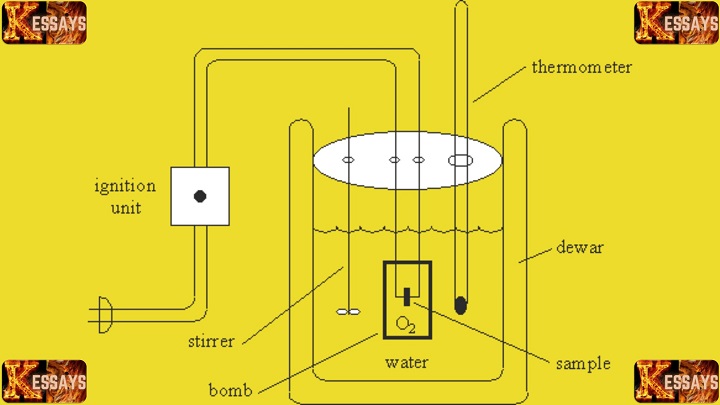
Introduction to the Calorimetry Lab Report
A Calorimetry Lab Report is a scientific examination of heat transfer that occurs during chemical reactions or physical changes. In this experiment, a device called a calorimeter is used to create a controlled and isolated environment. This allows students to measure temperature changes accurately and determine how much energy is absorbed or released by a system.
The main purpose of a Calorimetry Lab Report is not just to present numerical measurements. It also helps learners understand the thermodynamic principles that explain why energy moves the way it does. By observing temperature changes and calculating heat flow, students gain insight into essential concepts such as energy transformations, conservation of energy, and the relationship between heat, temperature, and matter.
This introduction prepares the learner to explore the key components of a calorimeter, the objectives of the experiment, the assumptions involved, and the potential sources of error. Ultimately, the Calorimetry Lab Report serves as both a practical lab exercise and a foundational learning tool for mastering core ideas in thermodynamics and experimental chemistry.

Core Principles of the Calorimetry Lab Report
The Calorimetry Lab Report is built on several key scientific principles. These ideas help students understand what is happening inside the calorimeter and why heat transfer occurs the way it does.
1. Heat Transfer: How Energy Moves
Heat transfer refers to how energy moves from one substance to another due to a difference in temperature.
In the calorimeter, heat always moves:
-
from the hotter substance → to the cooler substance
-
until both reach the same temperature (thermal equilibrium)
Understanding heat transfer helps students interpret temperature changes and calculate how much energy was gained or lost in a reaction.
2. Energy Transformations: How Energy Changes Form
During chemical reactions or physical processes, energy can change form.
For example:
-
Endothermic processes absorb heat
-
Exothermic processes release heat
The calorimeter captures these energy transformations by measuring temperature changes. These changes show whether the system gained or lost energy and by how much.
3. Thermodynamics: The Rules Governing Energy
Thermodynamics provides the scientific laws that explain why energy behaves the way it does.
In a Calorimetry Lab Report, students commonly apply:
-
First Law of Thermodynamics: Energy cannot be created or destroyed, only transferred or transformed.
This means the heat lost by one substance equals the heat gained by another inside the calorimeter. -
Conservation of Energy: Total energy within the system remains constant.
The calorimeter helps us observe this by isolating the system from outside influences.
These principles help students make sense of their measurements and calculations.
4. The Calorimeter: A Controlled Environment
The calorimeter is crucial because it isolates the reaction or process from the outside world.
It ensures:
-
minimal heat loss
-
accurate temperature readings
-
clear observation of energy flow within the system
By keeping conditions stable, the calorimeter allows students to analyze heat transfer with precision.
5. Temperature Change: Indicator of Energy Exchange
Temperature changes recorded inside the calorimeter directly indicate whether heat was absorbed or released.
A change in temperature means:
-
The system gained energy (temperature increase)
-
The system lost energy (temperature decrease)
These temperature readings form the basis for calculating heat and understanding energy transformations in the experiment.
In summary, the Core Principles of a Calorimetry Lab Report revolve around understanding how heat transfer, energy transformations, and thermodynamic laws interact within a controlled system. By observing temperature changes inside the calorimeter, students connect theoretical science to real experimental outcomes, gaining a deeper understanding of how energy moves and changes during chemical and physical processes.
Read Also: Bomb Calorimeter Lab Report Example
From Theory to Application
The Calorimetry Lab Report is not only about learning principles in theory. It also shows how heat transfer, energy transformations, and thermodynamics apply to real-world scientific problems.
1. Chemistry Applications
In chemistry, calorimetry helps measure the energy changes in reactions, such as:
-
Exothermic reactions releasing heat
-
Endothermic reactions absorbing heat
The calorimeter provides accurate temperature readings, allowing students to calculate the heat released or absorbed. This helps predict reaction behavior and understand chemical energy.
2. Environmental Science
Calorimetry is used to study energy flows in ecosystems and environmental processes. Examples include:
-
Measuring heat released during combustion of fuels
-
Studying energy efficiency in renewable resources
By applying thermodynamic principles, students can analyze how energy moves in environmental systems and impacts sustainability.
3. Materials Science
Materials scientists use calorimetry to test thermal properties of substances. This includes:
-
Heat capacity
-
Phase changes (melting, freezing, evaporation)
The Calorimetry Lab Report helps students understand how energy transformations occur in materials, which is crucial for designing safe and efficient products.
4. Biological Studies
In biology, calorimetry measures metabolic rates and energy expenditure in living organisms.
-
Heat produced during cellular respiration can be quantified
-
Energy efficiency of food and nutrient use can be analyzed
These studies rely on the same thermodynamic laws and principles of heat transfer observed in the calorimeter.
5. Translating Theory into Measurable Data
The Calorimetry Lab Report bridges the gap between abstract concepts and measurable outcomes. By recording temperature changes and calculating heat, students connect:
-
Theoretical thermodynamics → Experimental results
-
Energy concepts → Practical understanding of energy transformations
This demonstrates the practical value of calorimetry in multiple scientific disciplines.
In summary, the From Theory to Application section of the Calorimetry Lab Report shows that understanding heat transfer and energy transformations is essential across chemistry, environmental science, materials science, and biology. Using a calorimeter, students translate abstract thermodynamic principles into real, measurable data that explain energy behavior in diverse scientific fields.
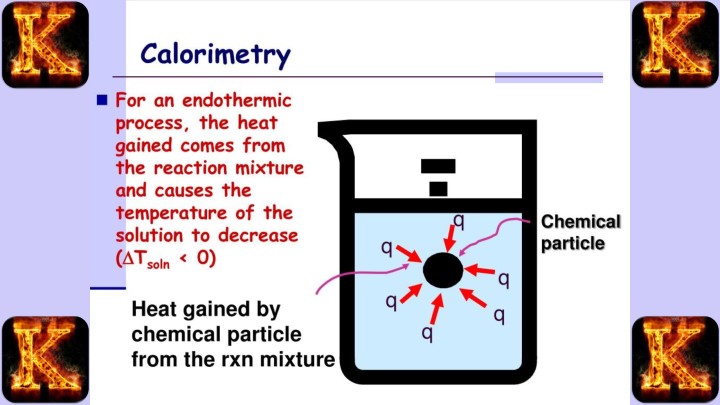
Purpose of the Calorimetry Lab
The Calorimetry Lab Report has a clear and focused purpose. It is designed to help students understand heat transfer, energy transformations, and thermodynamic principles through practical experimentation.
1. Quantifying Heat Changes
One of the primary goals of the lab is to measure how much heat is absorbed or released during a chemical reaction or physical process.
-
Exothermic processes release heat into the surroundings.
-
Endothermic processes absorb heat from the surroundings.
By measuring these changes with a calorimeter, students can calculate the exact amount of energy transferred in a system.
2. Analyzing Energetics of Reactions
The lab provides insight into the energetics of chemical or physical transformations. This includes understanding:
-
How energy moves within a system
-
How energy is transformed from one form to another
-
How these transformations obey the laws of thermodynamics
The Calorimetry Lab Report allows students to link theoretical concepts to experimental results.
3. Systematic Measurement and Observation
The calorimeter ensures that measurements are accurate and consistent. It isolates the system from external influences, which allows students to focus only on the heat changes caused by the reaction or process.
Systematic measurement involves:
-
Carefully recording temperature changes
-
Ensuring uniform mixing for consistent readings
-
Monitoring the system throughout the experiment
These steps help produce reliable data for analysis.
4. Accurate Interpretation of Results
Once data are collected, students analyze the results to determine:
-
The direction of heat transfer
-
The amount of energy transformed
-
Whether the experiment aligns with thermodynamic laws
Accurate interpretation develops critical thinking and reinforces understanding of how energy behaves in a controlled system.
In summary, the Purpose of the Calorimetry Lab is to provide a hands-on way to measure heat changes, analyze energy transformations, and apply thermodynamic principles in a controlled environment. Using a calorimeter, students learn to make precise observations, record systematic measurements, and interpret results accurately, bridging the gap between theory and real-world applications.
Read Also: Effects of Probiotics: Impact on Health and Well-being
Key Components and Objectives of the Calorimetry Lab Report
The Calorimetry Lab Report is designed to help students understand heat transfer, energy transformations, and thermodynamic principles through precise experimentation. Achieving these learning outcomes relies on three main objectives and the proper use of essential components in the lab.
Primary Objectives
-
Accurate Heat Measurement
The main goal is to determine how much heat is absorbed or released during a chemical or physical process. Recording precise temperature changes in the calorimeter allows students to calculate the exact energy exchanged in the system.
-
Understanding Energy Flow
Students explore how energy moves from one part of the system to another. Observing energy transformations within the calorimeter helps learners understand the behavior of heat in real reactions and how energy is conserved according to thermodynamic laws.
-
Interpreting Thermodynamic Behavior
By analyzing the data collected, students learn to interpret the results in the context of thermodynamics. This includes predicting whether reactions are endothermic or exothermic and understanding the broader energy changes occurring within the system.
Essential Components
-
Calorimeter
The calorimeter is the central instrument in the lab. It provides an isolated environment, minimizing heat loss to the surroundings. This ensures that all measured temperature changes accurately reflect the heat transfer occurring during the reaction or process. -
Thermometer
The thermometer records small but important temperature changes with precision. These readings are crucial for calculating energy transformations and understanding how heat moves within the system. -
Stirring Mechanism
The stirring mechanism ensures uniform heat distribution within the calorimeter. Without stirring, local temperature differences could lead to inaccurate measurements. Consistent mixing improves measurement reliability and supports accurate interpretation of thermodynamic behavior.
In summary, the Key Components and Objectives of a Calorimetry Lab Report work together to help students measure heat transfer, observe energy transformations, and apply thermodynamic principles. Using the calorimeter, thermometer, and stirring mechanism, learners can create controlled conditions that produce reliable data, enabling meaningful analysis and a deeper understanding of energy flow in chemical and physical processes.
Assumptions and Potential Errors
The Calorimetry Lab Report relies on certain assumptions to provide meaningful measurements of heat transfer, energy transformations, and thermodynamic behavior. Understanding these assumptions and potential sources of error helps students interpret results accurately and improve experimental reliability.
1. Assumptions
Every calorimetry experiment assumes ideal conditions to simplify the study of energy transformations. Common assumptions include:
-
Perfect insulation: The calorimeter is assumed to prevent heat from escaping to the environment.
-
Constant ambient conditions: Room temperature and surrounding conditions are assumed to remain stable.
-
Uniform energy distribution: Heat is assumed to be evenly distributed within the calorimeter.
These assumptions provide a controlled framework that makes it possible to connect temperature changes to heat transfer accurately.
2. Potential Errors
In practice, ideal conditions are rarely perfect. Several factors can introduce error in a calorimetry experiment:
-
Heat loss to the surroundings: Some energy may escape, reducing the measured temperature change.
-
Instrument limitations: Thermometers may have small inaccuracies or slow response times.
-
Inconsistent stirring: Uneven mixing can cause localized temperature differences, affecting heat measurements.
Recognizing these potential errors allows students to interpret their results realistically, rather than assuming perfect accuracy.
3. Importance of Acknowledging Assumptions and Errors
Acknowledging assumptions and potential errors does not undermine the experiment. Instead, it:
-
Highlights the limits of measurement accuracy
-
Encourages critical thinking in interpreting thermodynamic data
-
Supports continuous improvement in experimental design and scientific method
By understanding both assumptions and potential errors, students gain a deeper appreciation of how heat transfer and energy transformations are measured and analyzed in a controlled calorimeter setting.
In summary, the Assumptions and Potential Errors section of a Calorimetry Lab Report helps students understand the ideal conditions under which heat transfer and energy transformations are studied. Awareness of potential errors, such as heat loss or instrument limitations, strengthens the reliability of interpretations and encourages careful experimental practice, reinforcing both practical skills and understanding of thermodynamic principles.
Read Also: Applying System Dynamics in Carbon Credit Trading
Abstract and Results
The Calorimetry Lab Report uses the abstract and results sections to clearly communicate the purpose, methodology, and findings of the experiment. These sections help students understand heat transfer, energy transformations, and thermodynamic behavior in a structured way.
1. Abstract
The abstract provides a concise summary of the Calorimetry Lab Report. It includes:
-
Purpose of the experiment: Explaining what is being studied, such as measuring heat transfer during a chemical or physical process.
-
Methodology: Describing the use of the calorimeter, thermometers, and other tools to monitor temperature changes.
-
Key findings: Highlighting the main results, including observed energy transformations and calculated heat values.
The abstract helps readers quickly understand what the experiment is about and what to expect in the full report.
2. Results
The results section presents the numerical data collected during the experiment. This includes:
-
Temperature changes measured in the calorimeter
-
Heat values calculated from observed data
-
Energy measurements that show the extent of energy transformations
The results provide concrete evidence of thermodynamic behavior and allow students to analyze the efficiency and direction of heat transfer.
3. Linking Abstract and Results
The abstract and results sections work together to provide a clear narrative:
-
The abstract introduces the experiment and highlights key findings
-
The results give detailed, measurable evidence that supports those findings
This structure ensures that the Calorimetry Lab Report is easy to follow, guiding readers from a general overview to detailed experimental data.
In summary, the Abstract and Results section of a Calorimetry Lab Report helps students communicate and interpret heat transfer, energy transformations, and thermodynamic principles. The abstract gives a brief, accessible overview, while the results present detailed calorimeter measurements and calculations. Together, they create a clear framework for understanding the experiment from concept to evidence.
Navigating the Lab Report
To navigate the intricacies of the Calorimetry lab report effectively, students can seek guidance from reputable assignment help websites such as kessays.com and peachyessays.com. These platforms provide valuable insights, exemplary samples, and expert assistance, empowering students to craft well-structured, insightful analyses of their Calorimetry lab experiments. As we embark on the journey through this guide, the focus remains on unraveling the nuances of calorimetry, guiding students toward a comprehensive understanding of this indispensable scientific technique.